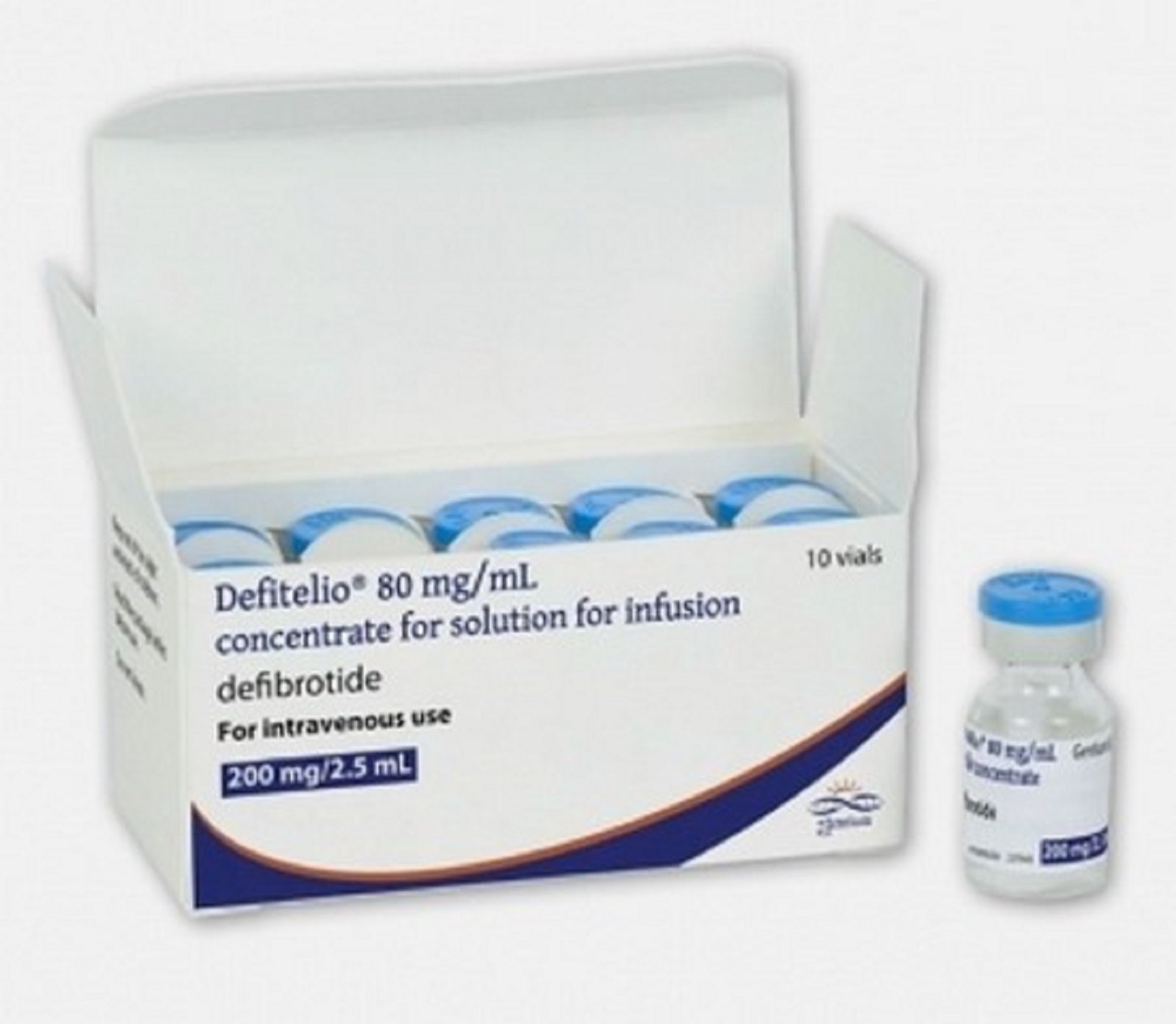
Geneva: The World Health Organization (WHO) Medical Product Alert today referred to a falsified batch of DEFITELIO (defibrotide sodium), detected in India (April 2023) and Türkiye (July 2023) and was supplied outside of regulated and authorized channels.
DEFITELIO does not have marketing authorisation in India and Türkiye. WHO said the use of falsified DEFITELIO will result in the ineffective treatment of patients and may pose other serious risks to health because of its intravenous administration and could be life-threatening in some circumstances. It said it was not currently aware of any reports of adverse events following the use of this reported falsified DEFITELIO, however, the safety, sterility, and quality of the falsified products referenced in this alert were unknown.
DEFITELIO (defibrotide) is indicated for the treatment of severe hepatic veno-occlusive disease (VOD) which is a condition in which the veins in the liver become blocked and hence stop the liver from working correctly. It is indicated for adults, adolescents, children and infants over 1 month of age.
VOD is also known as sinusoidal obstructive syndrome (SOS) in haematopoietic stem-cell transplantation (HSCT) therapy.
“The genuine manufacturer of DEFITELIO has confirmed that the product referenced in this Alert is falsified,” WHO stated. It referred to the “genuine” manufacturer advising that:
- Genuine DEFITELIO with Lot 20G20A was packaged in German/Austrian packaging.
- The falsified products instead are in UK/Ireland packaging.
- The stated expiry date is falsified and does not comply with the registered shelf life.
- The stated serial number is not associated with batch 20G20A.
- DEFITELIO does not have marketing authorization in India and Türkiye.
WHO had previously issued Alerts for falsified DEFITELIO detected in other countries too. In its advice to regulatory authorities and the public, WHO said. “If you have any of the affected products, WHO recommends that you do not use them. If you, or someone you know, has or may have used the affected product, or suffered an adverse reaction or unexpected side-effect after use, you are advised to seek immediate medical advice from a healthcare professional. Healthcare professionals to report the incident to the National Regulatory Authorities/National Pharmacovigilance Centre. National regulatory/health authorities are advised to immediately notify WHO if they identify these falsified products.”
WHO stressed that all medical products must be obtained from authorized and licensed suppliers. “If you have any information about the manufacture or supply of these products, please contact WHO via rapidalert@who.int,” it stated.
-global bihari bureau