Zweli Mkhize
Pretoria: South African Health Minister, Zweli Mkhize, said on Sunday that the country would suspend its roll out of the Oxford/AstraZeneca vaccines after studies showed that it did not protect clinical trial participants from the more contagious variant of the virus first detected there.

Mkhize said he was taking advise of scientists on the best way to proceed, after AstraZeneca’s announcement that its vaccine only offered limited protection against mild disease caused by the variant, known as B.1.351, that has been discovered in South Africa. Scientists on a televised programme, said that those infected by earlier versions of the virus were apparently not protected from the new Coronavirus variant.
Incidentally, South Africa was one of the countries where clinical trials were held to assess the Oxford/AstraZeneca drug’s efficacy.
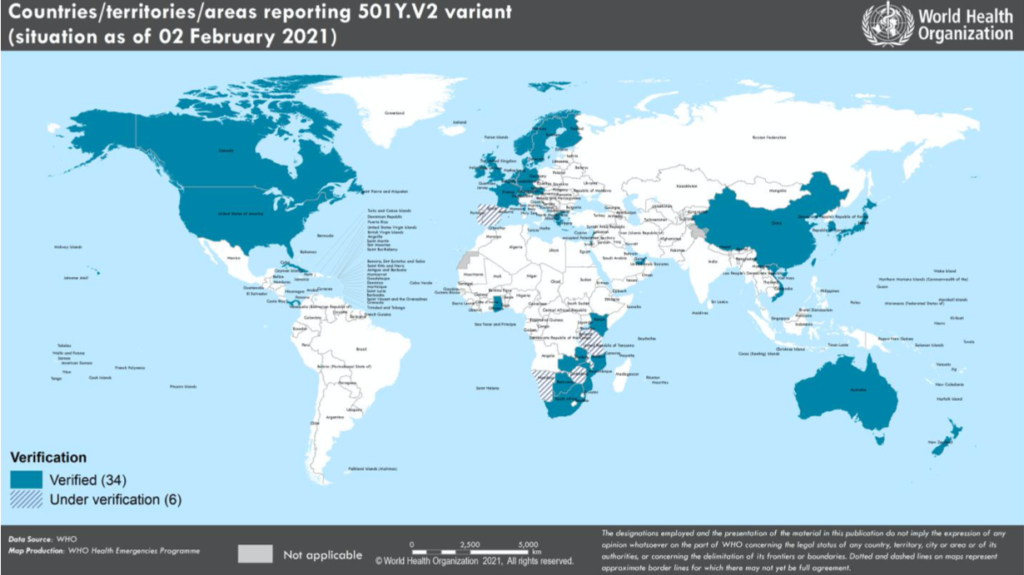
This is no good news in the combat against the pandemic. Between the period January 27 and February 2, 2021, the Variant 501Y.V2, lineage B.1.351 has been reported from ten additional countries– now totaling 41 countries across four of the six WHO regions. (see figure below)
As it is, the South African government’s decision is a severe blow to the country’s efforts to tackle the pandemic and where Variant 501Y.V2 is now widespread across the country. South Africa has bought 1.5 million doses of the AstraZeneca vaccine from the Serum Institute in India of which a million doses arrived in the country last week and the rollout of the vaccines to inoculate healthcare workers was to start this week.
On February 4 this year when South African President Cyril Ramaphosa had a telephonic conversation with Indian Prime Minister Narendra Modi following the arrival in South Africa of the first batch of 1 million doses of COVID-19 vaccines from the Serum Institute in India on Monday, 1 February 2021, President Ramaphosa had applauded the Government and people of India for its gift to the world in the form of vaccines and scientific knowledge. He had conveyed the profound gratitude expressed by the people of South Africa to India for its solidarity with South Africa in fighting the pandemic. During the conversation, Prime Minister Modi had also indicated that India would be developing one or two more vaccines and would continue to support countries in Africa with a special package of vaccines. President Cyril: Developments in response to Coronavirus COVID-19.
When the vaccines had arrived in South Africa on February 1, 2021, Ramaphosa had chosen the occasion to address the nation to announce: “Earlier today the Deputy President David Mabuza, Minister of Health Dr Zwelini Mkhize, Minister Khumbudzo Ntshavheni and I, received our country’s first consignment of COVID-19 vaccines. The consignment, consisting of one million doses of the Covishield vaccine produced by the Serum Institute in India, arrived at OR Tambo International Airport this afternoon.”
The hope that these vaccines had generated could be reflected in these words of Ramaphosa: “The arrival of these vaccines contains the promise that we can turn the tide on this disease that has caused so much devastation and hardship in our country and across the world.
After their arrival, the vaccines were tested at the National Control Laboratory in South Africa to confirm that their integrity had been maintained during transportation. Another 500,000 doses from the Serum Institute of India was to arrive later in February.
Incidentally, in addition to the 1 million Covishield doses that South Africa had received from India, it has also secured 12 million doses in total from the global COVAX facility, which has indicated that it will release approximately 2 million doses by March.
While the country has secured 9 million vaccine doses from Johnson & Johnson, commencing with delivery in the second quarter, it has secured Pfizer’s commitment for 20 million vaccine doses commencing with deliveries in the second quarter.
– global bihari bureau