COVID-19 Notes
 By Dr. Satish K Gupta*
By Dr. Satish K Gupta*
Booster Dose Mix And Match
Should the 3rd dose booster be of same vaccine as primary series or a different one?
What should be the optimal desired interval of third dose booster?
COVID Update: 1,79,723 new cases in the last 24 hours till 9 am today
Daily positivity rate (13.29%)
Mix and Match (heterogeneous Third dose booster after primary series)
As per Government of India:
There will be no mix-and-match of vaccines for those eligible to get a ‘precautionary’ third dose.
This means individuals who received two doses of the Serum Institute’s Covishield will get the same vaccine this time, and those who got Bharat Biotech’s Covaxin will get a third jab of that vaccine.
Why Some health care workers are pitching for a third dose booster with different vaccine?
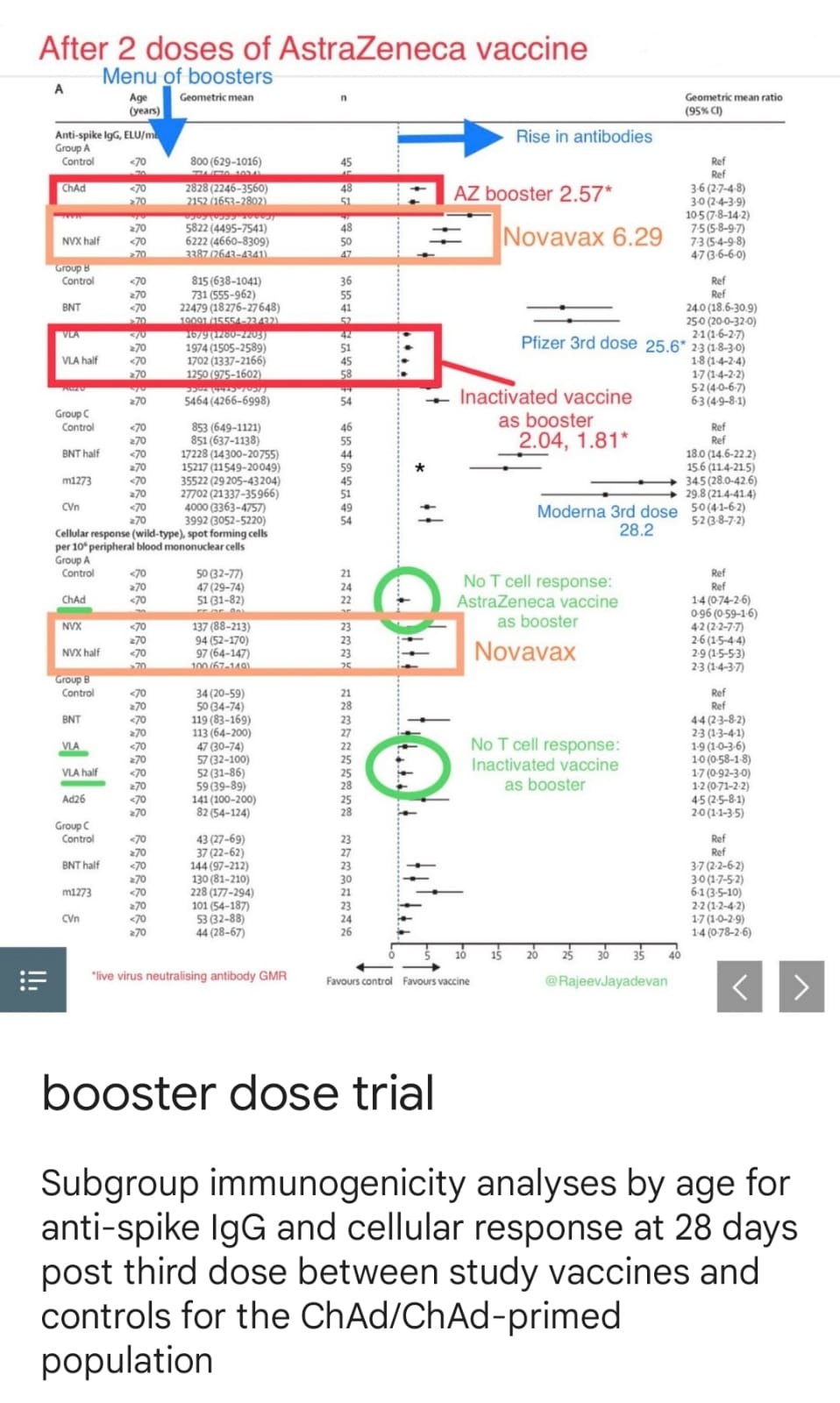
CovBoost Trial. A double blind excellent study funded by UK Vaccine Taskforce and National Institute for Health Research published on December 2, 2021 found that third dose booster by a different vaccine boosted the immunity to much higher levels especially after primary vaccination with Killed vaccine or Vector vaccine (Chadox1).
As per the study all vaccines boosted the immunity after Chadox1 but there were marked differences in response. For example:
AstraZenaca used as booster increased levels of neutralizing antibodies to 2.5 times while Novavax vaccine raised it to 6.2 times.
Killed virus vaccine, Valneva, from Europe boosted antibodies 2.04 times.
Note: Covboost trial did not include Covaxin. But another killed vaccine Valneva manufactured in Europe.
See Fig.(With inputs from Dr Anupam Singh, EDPA)
(https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02717-3/fulltext)
Also it is pertinent to know that study was done in UK and results may vary in different populations.
Also note that the UK COMCOV trial was heterologous prime boost trial related to *second dose* of vaccine. It demonstrated that a heterologous prime-boost schedule can be more immunogenic than a homologous schedule, albeit with increased reactogenicity in some combinations. (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01694-9/fulltext)
Why is the Government not offering Novavax as third Dose booster?
Despite huge availability of doses of Novavax (Covovax) in India, why the Government advisers choose to stick with homologous booster could be something difficult to understand. One possible reason could be absence of booster dose trials with Covovax in Indian subsets. However going by study results of CovBoost trial Covovax could have been a wonderful choice as a third dose booster.
World Health Organization’s stand on mix and match of Novavax
Though WHO accepts two heterologous doses of its COVID-19 vaccines as a complete primary series. However, there is limited evidence available on the use of Novavax (NVX-CoV2373) in a heterologous schedule.
Is Novavax approved?
Yes, it is Approved for emergency use EUL by WHO, European Medical Agency and DCGA India. FDA has not approved Novavax as yet.
Let’s once see
What are other countries offering as third dose booster? Mix and Match or same/similar vaccine?
The United Kingdom
●To all more than 18 year plus
●Booster dose at least 3 months after the 2nd dose.
●Most people are offered a booster dose of the Pfizer/BioNTech or Moderna vaccine irrespective of primary vaccines.
●Some people may be offered a booster dose of the Oxford/AstraZeneca vaccine if they cannot have the Pfizer/BioNTech or Moderna vaccine.
The United States of America
●Booster to all more than 18 yr of age
●6 months after the primary series of Pfizer/Moderna
●Same vaccine plateform
●If more than 18 yrs and Johnson vaccine was taken in primary vaccine then booster offered 2mths after and would be from either Pfizer/Moderna.
Switzerland
●Using same vaccine as third dose booster as primary vaccine
Spain
●Spain had administered 9.76 million booster shots until December 16, 2021.
So most of developed countries are offering Pfizer or Moderna vaccine as third dose booster which remains the same vaccine as the primary series in most cases except in people who received single dose J&J vaccine (Ad26) or two sides of Chadox1 vaccine.
No country has yet mounted Novavax as third dose booster despite evidence from CovBoost trial, not even UK where trial was done. It continues to offer Chadox1 to some people who received it earlier as well.
Time interval controversy with third dose booster. What should be the optimal desired interval of third dose booster?
India is offering third dose booster after Nine months (39 wks) of second dose, while in the USA, the cut off period is 6 months. Brazil is giving it after 5 months, and in the UK, it is 3 months.
France on December 24, 2021 recommended booster vaccination after three months reducing the guideline of five months to better fight the Omicron variant.
How to decide the interval of third dose booster?
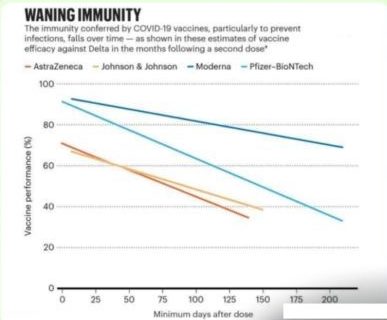
Studies have shown that the effectiveness falls to just 34 percent four months after vaccination with the Pfizer vaccine but the efficacy rose to 75 percent two weeks after a booster dose.
It is noteworthy that different vaccines will have different duration of effectiveness as measured by neutralizing antibody titres. So Govts ought to decide the interval of third dose booster based on waning immunity after second dose. Other important deciding factors could be availablity of doses.
For India the decision of using same vaccine rather than mix and match of Covaxin and Covishileld could have been driven by following factors:
1. Absence of Indian trial data on mix and match using Covishield and Covaxin
2. Limitation of dose supply of Covaxin. HCW FLW constitute 3 crore people and 60 yr plus with comorbidities people constitute another 2.7 Crore and ninety percent of them having received Covishiled in primary series could have become eligible for Covaxin in case mix and match allowed. Covaxin presrntly being committed for 15 to 18yr age group already had a booked demand of 7.3 crore doses. Seeing current and future supply constraints of Covaxin it was in best interest of nation to follow same Third Dose Booster.
It is noteworthy that covaxin is manufactured in BSL4 labs and there are very few labs in world fulfilling the BSL4 criteria.
Let’s not get worried
Does cell mediated immunity come to the rescue?
As stated earlier the concept of third dose booster is primarily driven by Neutralizing Antibody titres but vaccines remain effective despite decreasing antibody titres. Most of us tend to forget the role of cell mediated immunity just because there are no commercially available tests to measure the same.
T cell immunity is not affected by spike protein mutations in virus.
T cells are important in controlling disease severity. T-cell activity appears minimally affected by spike antigen mutations and responses remain effective even against variants of concern, even though neutralising antibody levels are reduced. As per the studies although neutralising antibody titres after mRNA vaccination may be higher compared to adenovirus vector vaccines, there are much smaller differences in T-cell responses.
Are there any trials with Covaxin as third dose booster in India?
Yes. A phase 2, double-blind, randomised controlled trial demonstrated long-term safety of Covaxin vaccine 6 months after the primary series.
Analysis showed that six months after a two-dose BBV152 vaccination series, cell-mediated immunity and neutralising antibodies to both homologous (D614G) and heterologous strains (Alpha, Beta, Delta, and Delta plus) persisted above baseline, although the magnitude of the responses had declined.
Furthermore, neutralising antibodies against homologous and heterologous SARS-CoV-2 variants increased 19 to 265 fold after a third vaccination. Booster by Covaxin (BBV152) vaccination is safe and may be necessary to ensure persistent immunity to prevent breakthrough infections. (https://www.medrxiv.org/content/10.1101/2022.01.05.22268777v1)
Booster dose trial with AstraZeneca?
Booster dose trial from Chadox1 (Covishield):
A sub-analysis from the COV001 and COV002 trials demonstrated that a third dose of Vaxzevria given at least six months after a second dose boosted antibody levels six-fold and maintained T cell response. A third dose also resulted in higher neutralising activity against the Alpha, Beta, and Delta variants, compared with a two-dose regimen. In the trial, the third dose of Vaxzevria was less reactogenic than the first dose.
In addition, the COV-BOOST trial showed that a third dose booster of Vaxzevria induced significantly higher immune responses compared with controls against the Delta variant and original strain following a primary vaccine series of Vaxzevria or Pfizer BioNtech (BNT162b2). (https://www.astrazeneca.com/media-centre/press-releases/2021/vaxzevria-significantly-boosted-antibody-levels-against-omicron.html#!)
Will the same third dose booster as offered by the Government be effective?
Yes. Government has moved a step forward to offer the third dose booster. There could be controversies regarding timing, type of vaccine, immunogenicity but it is safe and should be effective as immunity booster.
Decrease in cases of breakthrough infection among Health Care Workers (HCW) in coming days will be the real test of efficacy of any booster. Nevertheless, it will serve as wonderful Moral Booster.
If you are too much trial driven and evidence savvy please don’t forget that even placebos have an efficacy of more than 33%.
Also note that taking the same booster makes you eligible to get certificate of 3rd dose booster, which might be useful in times to come.
Also read: How effective will be the booster dose that rolls out from tomorrow?
*Dr. Satish K Gupta is an MD in Medicines, a Visiting Senior Consultant Physician and Internist at Max Super Speciality Hospital, and a Clinical Assistant Professor at GS Medical College, Chaudhary Charan Singh University, Meerut. He is the author of Journey of COVID in India: A Doctor’s Perspective.





