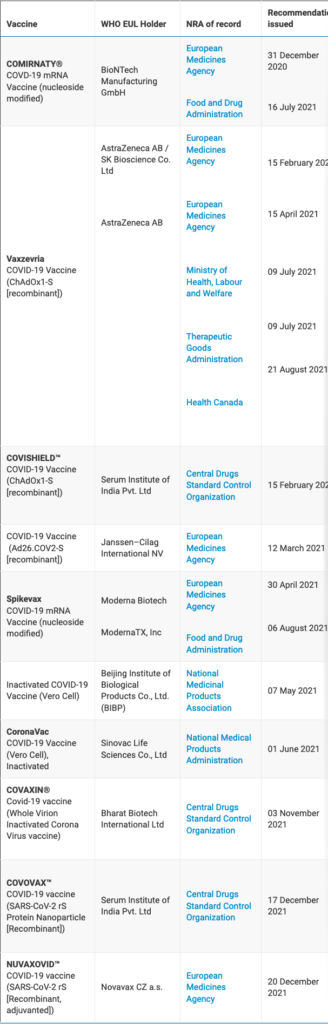
Geneva: The World Health Organization issued an emergency use listing (EUL) for Nuvaxovid™, following its assessment and approval by the European Medicines Agency (EMA) earlier today.
The new vaccine was developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI), and is the originator product for the Covovax™ vaccine that received WHO emergency use listing on 17 December.
Both vaccines are made using the same technologies. They require two doses and are stable at 2 to 8 °C refrigerated temperatures.
WHO’s Strategic Advisory Group of Experts on Immunization has also issued policy recommendations for Nuvaxovid™ / Covovax™.
The emergency use listing (EUL) procedure assesses the suitability of novel health products during public health emergencies.
As part of the EUL process, the company producing the vaccine must commit to continue to generate data to enable full licensure and WHO prequalification of the vaccine. The WHO prequalification process will assess additional clinical data generated from vaccine trials and deployment on a rolling basis to ensure the vaccine meets the necessary standards of quality, safety and efficacy for broader availability.
– global bihari bureau