Special Focus
Geneva: The World Health Organisation today stated that it was closely monitoring the public health events associated with SARS-CoV-2 variants. Providing an update on ongoing studies and the geographical distribution of three variants of concern (VOCs) as reported by countries, territories and areas (hereafter countries) as of 8 February 2021, it mentioned that since the last update, an additional 6 countries had reported cases of variants VOC202012/01, 3 additional countries reported variant 501Y.V2, and 5 additional countries reported variant P.1 (Table 3, Figures 3,5,7).
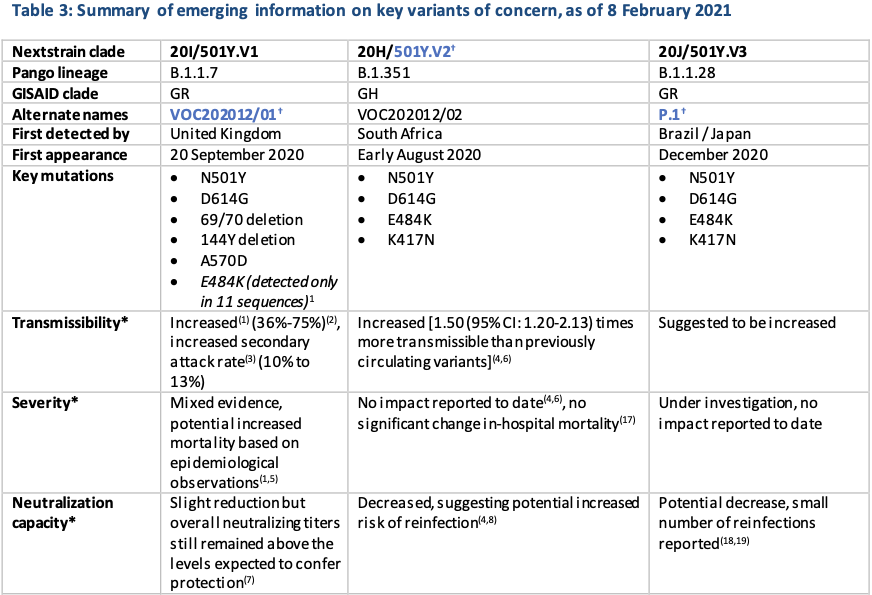
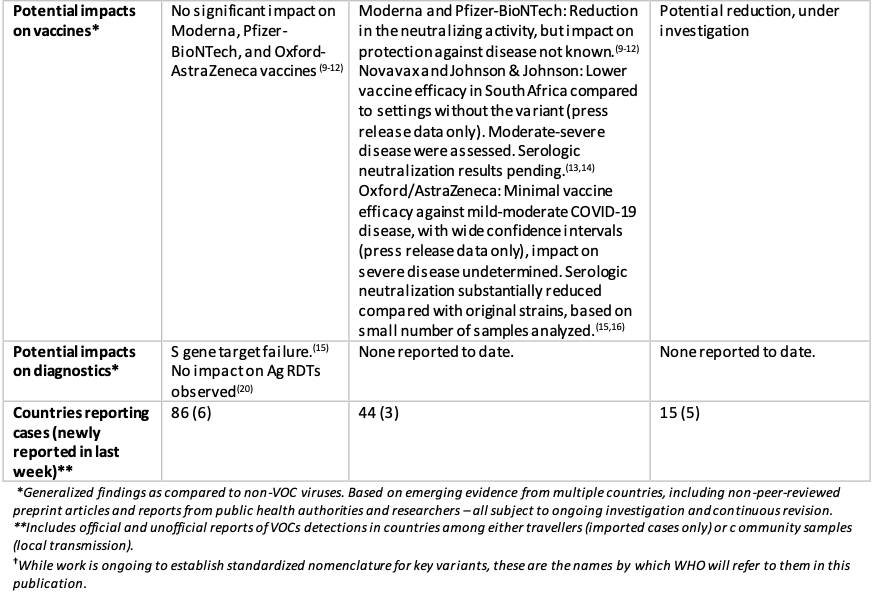
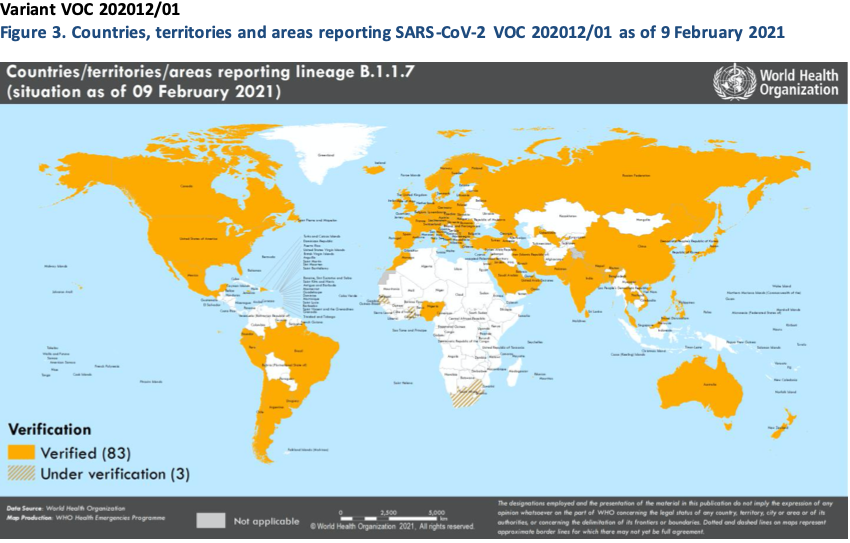
Local transmission of VOC202012/01 has been reported in a growing number of countries in the European Region and in some areas of North America. The VOC 202012/01 variant has shown increased transmissibility, including increased secondary attack rates, and some evidence of increase in disease severity. More recently, results from a Phase 3 trial conducted by Novavax demonstrated an efficacy of 85.6% against this variant in the United Kingdom.
While preliminary studies showed post-vaccination sera with Pfizer-BioNTech and Moderna vaccines had limited to no significant change against the VOC202012/01 variant, recently, the E484K mutation in the spike protein had been detected in 11 sequences within the B.1.1.7 lineage in the United Kingdom. This mutation was also found in 501Y.V2 and P.1 variant, but the three variants had arisen separately and were not linked to each other. Mutation E484K had been identified as an “escape mutation,” which had shown the ability to reduce the neutralising activity by monoclonal antibodies or convalescent sera. A preliminary study had shown further reduction in neutralisation activity by vaccine elicited antibodies if E484K mutation was present alongside the VOC202012/01 variant. The detected E484K mutation within this lineage was currently limited to a small number of cases, and these are all preliminary findings which require further investigation involving larger sample sizes.
In the United Kingdom where this variant was initially identified, the proportion of cases with VOC202012/01 among tested samples had increased from 63% in week commencing December 14, to 90% in week beginning January 18, 2021. This high rate of detection of VOC202012/01 had persisted in recent weeks while the case and death counts were showing a declining trend. From 11 January through February 7, a decreasing trend had been observed, following the implementation of stringent public health and social measures.
Similarly, in other European countries such as Ireland and Denmark, a marked increasing trend in the number of new COVID-19 cases was detected in late December 2020 as the countries were reporting local transmission of VOC202012/01. In Ireland, local authorities have reported the proportion of cases with VOC202012/01 among tested samples reached over 63% in the week starting on the 25 January and over 7% in Denmark the week starting the 11 January 2021. Implementation of more intensive public health and social measures at the end of December and beginning of January led to marked declines in COVID-19 case and death incidence in both countries.
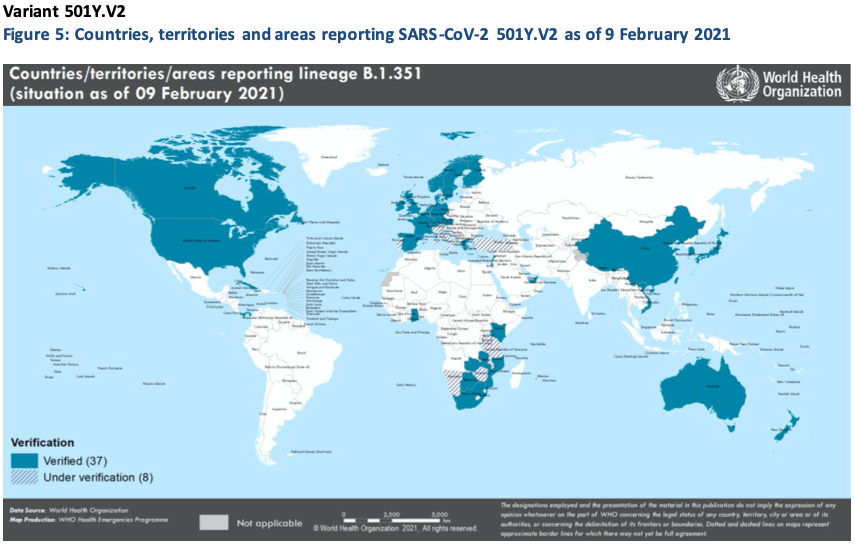
There is evidence to suggest that 501Y.V2 transmission is occurring in several countries in the African Region, with clusters of cases or ongoing local transmission suggested in countries in other regions.
In South Africa, where this variant was initially identified, a progressive decreasing trend in case and death incidence, has been observed following the implementation of stringent Public health and social measures (PHSM). Here, studies have shown that the second wave (predominated by 501Y.V2 circulation) was associated with a higher incidence, faster increases in cases and hospitalizations, and increased mortality risk in weeks with high rates of hospital admission reflecting increased pressure on the health system. However, it was not associated with increased in-hospital mortality (17) – suggesting disease severity may be similar to previously circulating variants.
The 501Y.V2 variant has shown increased transmissibility, and laboratory-based studies noted a small reduction in the neutralising activity against SARS-CoV-2 501Y.V2 variants in individuals vaccinated with the Moderna or Pfizer-BioNTech vaccines, although the neutralising titers still remained above the levels expected to confer protection.
New preliminary results of Novavax, Johnson & Johnson, and Oxford/AstraZeneca vaccines have shown potential reduced effectiveness against 501Y.V2. Phase 3 trials of the Johnson & Johnson vaccine found 66% effectiveness in preventing moderate to severe infections, 28 days after vaccination; however, the efficacy varied across the three trial locations: the South Africa efficacy (57%) was lowest, and reflects 95% of the disease causing strains were the variant.
Similar preliminary results from Novavax had shown 60% efficacy against 501Y.V2 . In a small trial of approximately 2200 subjects in South Africa, a two-dose regimen of the Oxford/AstraZeneca vaccine resulted in a non-significant efficacy of 21.9% against mild- moderate COVID-19 which included a period when the majority of cases were caused by 501Y.V2; however, efficacy against severe COVID-19, hospitalisations and deaths was not studied.
Serologic neutralisation was substantially reduced compared with original strains, based on small number of samples analysed. Notably, primary analysis of data from Phase III trials in the context of viral settings without this variant had shown that the AstraZeneca/Oxford vaccine offers protection against severe disease, hospitalisation and death; therefore, it remains vitaldly important to determine the vaccine’s effectiveness for preventing more severe illness caused by the 501Y.V2 variant.
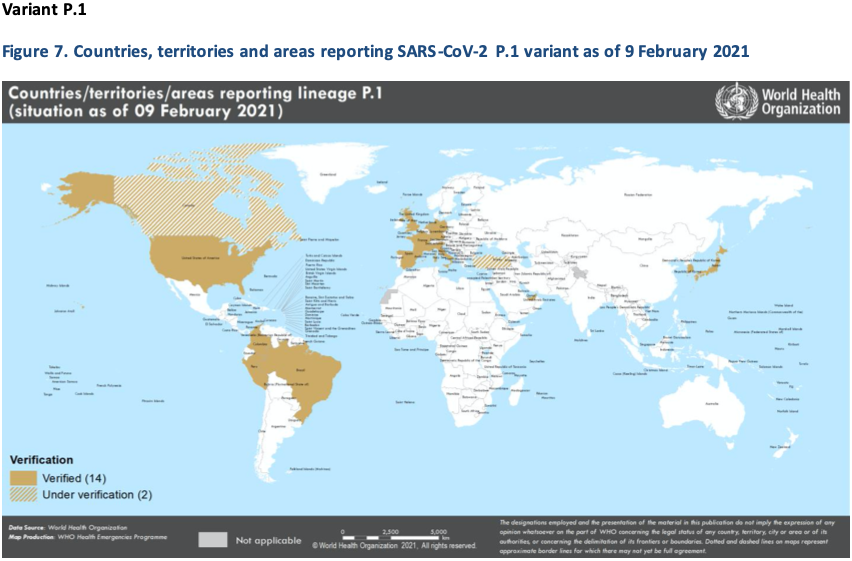
In Brazil, where the P.1 variant was initially identified in addition to detection in a group of travellers from Brazil to Japan, a second wave of cases and corresponding deaths was observed with increasing trends beginning late November 2020, but has shown early signs of waning this week (Figure 8). In Manaus, Brazil, the proportion of cases with P.1 among tested samples have increased from 52% in December 2020 to 85% in January 2021 (23). Based on preliminary investigations, the mutations detected in P.1 variant could potentially reduce antibody neutralization (18); however, additional studies are required to assess if there are changes in transmissibility, severity or antibody neutralizing activity as a result of this new variants.
It is important to note that these are preliminary findings which require further investigation including the need for assessment of vaccine performance against severe disease, assessment of neutralising activity in a larger number of samples and for other vaccines against this strain, an evaluation of changes in neutralisation on clinical efficacy and eventually, an estimate of the effectiveness of these vaccines on the current emerging variants.
Manufacturers are concurrently exploring potential ways to improve protection against emerging VOCs, such as augmenting dosages and dosage intervals, introducing booster doses or booster vaccines, and beginning work to adapt vaccines and optimize production pipelines to allow for rapid strain changes, should this become necessary.
Source: COVID-19 Weekly Epidemiological Update Data as received by WHO from national authorities, as of 7 February 2021.
– global bihari bureau




A person essentially lend a hand to make significantly posts I
might state. This is the first time I frequented your
web page and thus far? I surprised with the analysis you made to
create this particular submit amazing. Great process!