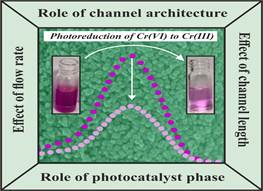
Mohali: A low-cost method to remove toxic Chromium from wastewater of industries such as leather tanning and electroplating by using sunlight as a catalyst in combination with microfluidic technology has been developed by researchers at the Institute of Nano Science and Technology (INST), here.
Toxicity of hexavalent chromium is a serious concern and as per reports by WHO the tolerable concentrations of hexavalent and trivalent chromium in drinking water are limited to 0.05 mg/L and 5 mg/L. Thus, it becomes imperative to reduce this hexavalent form of chromium to the trivalent form.
Several chemical and physiochemical methods, such as ion exchange, adsorption, and bacterial and chemical reduction, have been employed for the removal of Chromium (VI). Most of these techniques are costly, with low removal efficiencies of Chromium (VI).
Dr Bhanu Prakash’s research group from INST developed a new technique of removing toxic Chromium (VI) ions by utilizing sunlight for the catalytic process in combination with microfluidic technology for the conversion of the toxic hexavalent form of chromium to a less toxic trivalent form. They used a process called continuous flow photoreduction and validated this process in wastewater using TiO2 nanoparticles with the help of a smartphone-based colourimetric technique.
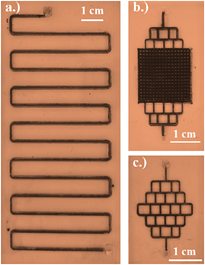
Besides, the cost-effectiveness of the process and the usage of renewable energy, with the microfluidics route, the reduction efficiency can also be tailored by fine-tuning the flow rate of the organic pollutant, reactor dimension, and architecture, precisely. One of the most advantageous features of using microreactors is the reusability of the photocatalyst without any recovery agents or cumbersome processes.
Various microfluidic parameters such as reactor design, flow rate and channel length along with different catalyst phases were fine-tuned to bring about superior degradation efficiency. Superior degradation efficiency of 95 % was attained by utilizing a serpentine microreactor coated with a photocatalyst in the pure anatase phase at a flow rate of 50 µl/min.
The researchers started the process with the fabrication of microfluidic reactors and the synthesis of nanocatalysts. Next, the nanocatalyst was immobilized onto the microreactor bed and flow experiments were performed. The extent of conversion was monitored using a change in absorbance via UV-Vis spectroscopy. This was followed by evaluating the reactor performance on basics of long-term stability of microreactor and photocatalyst with respect to the number of cycles or volume processed.
This work published in the Chemical Engineering Journal holds potential in industrial translation by increasing the throughput of the approach. This is possible by setting up microfluidic reactors in a parallel approach (arrays) or by microtexturing the bulk reactor surface to increase the efficacy of the process after repetitive use.
– global bihari bureau
Topmost image by Marion Wellmann from Pixabay