
India Secures Unanimous Mandate at CAC48; Champions Trade, Safety, and Quality
Rome: India was today re-elected to the Executive Committee (CCEXEC) of Codex for the Asia region during the 48th session of the Codex Alimentarius Commission (CAC48), securing a unanimous mandate that underscores its collaborative leadership in global food governance. The endorsement ensures that the technical and trade priorities of the Asian continent will be represented at the highest level until the end of CAC50 in 2027, positioning India to influence both regulatory frameworks and market access for regional exporters.
The Codex Alimentarius Commission (Codex) is the United Nations-backed international body responsible for developing food standards, guidelines, and codes of practice to ensure food safety, quality, and fair trade globally. Unlike a new appointment, India’s reelection is significant because it consolidates continuity and strengthens India’s mandate on ongoing initiatives. The unanimous support from all member countries reinforces India’s credibility, enabling it to advance critical projects—such as the cashew kernel standard, fresh fruits and curry leaves standards, aflatoxin management, and data modernisation—without interruption. India’s leadership was particularly visible during the CCEXEC89 session earlier in the week, where, as Member Asia, it strongly contributed to discussions on Codex efficiency, future challenges, and strategic priorities, giving it greater leverage to ensure regional perspectives are incorporated into global decision-making. This reelection is not just a formal continuation but a reaffirmation of India’s leadership and strategic influence within global food governance.

The Indian delegation, led by Rajit Punhani, Chief Executive Officer of the Food Safety and Standards Authority of India (FSSAI), included officials from the Ministry of Health and Family Welfare, as well as technical expert organisations. India actively shaped discussions on improving Codex efficiency, data management, and equitable standard-setting. Delegates emphasised the need to update databases on food additives, pesticide residues, veterinary drugs, methods of analysis, and contaminants, while promoting the use of artificial intelligence and modern technologies to enhance translation, review, and operational efficiency.
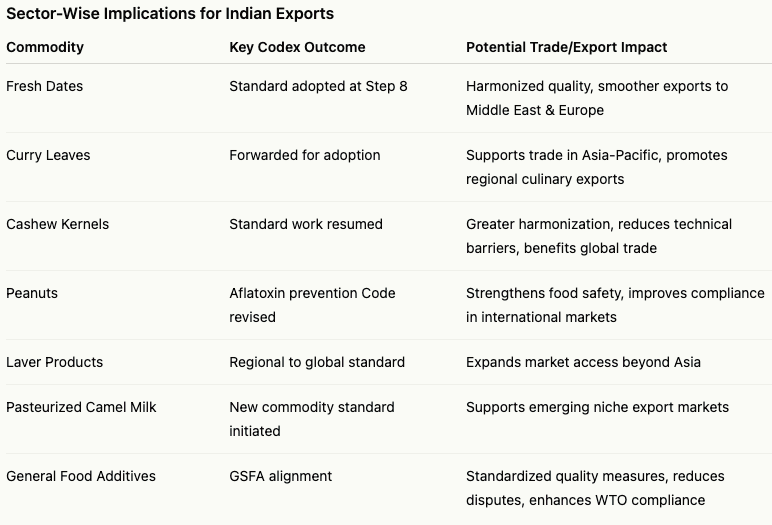
India’s impact was visible across multiple Codex committees. At the Codex Committee on Fresh Fruits and Vegetables (CCFFV), India chaired working groups where the Standard for fresh dates was adopted at Step 8, harmonising trade practices and raising quality benchmarks for a globally significant fruit. The Standard for fresh curry leaves, a regionally important culinary herb, was also forwarded for adoption, supporting trade in the Asia-Pacific region. At the Codex Committee on Pesticide Residues (CCPR), India contributed to guidelines for monitoring the stability and purity of reference materials, bolstering laboratory capacity and ensuring the reliability of data used in setting Maximum Residue Limits (MRLs). India also contributed to revisions in the Code of Practice for the prevention of aflatoxin contamination in peanuts and helped align food additive provisions in the General Standard for Food Additives (GSFA). At the Codex Committee on Methods of Analysis and Sampling (CCMAS), India secured inclusion of “Nitrogen to protein conversion factors” as an annexe, providing a standardised tool for compositional assessment across food sectors, benefiting laboratories regionally and internationally. Across all committees, India ensured that global standards remained relevant and practical for the Asian region, demonstrating leadership in bridging global standard-setting with regional applicability.
A key trade-related achievement was the advancement of the Standard for Cashew Kernels, a globally traded commodity for which India is a major producer and exporter. India successfully advocated resuming work on this standard, with CAC48 recommending issuance of a Circular Letter to collect comments before review at CCEXEC90 and consideration at CAC49. India also supported converting the regional standard for laver products into a worldwide standard and welcomed new work on a commodity standard for pasteurised liquid camel milk, reflecting growing regional influence and forward-looking trade strategy.
For Indian exporters, these outcomes have practical implications. Harmonised standards reduce trade friction, enhance predictability in international markets, and ensure that products meet Codex-aligned quality requirements, which are often referenced in World Trade Organization (WTO) trade disputes. Fresh fruits, curry leaves, cashew kernels, and other commodities now have clearer pathways for market acceptance, lowering technical trade barriers and potentially boosting export volumes and revenue. Adoption of standardised testing methods and improved reference materials also strengthens India’s laboratory network, ensuring compliance and credibility in international transactions.
The session reaffirmed India’s commitment to multilateral collaboration and equitable food governance. The delegation included senior officials from the Ministry of Health & Family Welfare, Ministry of Commerce & Industry, Spices Board, Marine Products Export Development Authority (MPEDA), ICMR-National Institute of Nutrition (NIN), Indian Council of Agricultural Research (ICAR), and the Federation of Indian Chambers of Commerce and Industry (FICCI), underscoring a cross-sectoral and integrated approach to both regulatory and trade priorities.
Strategically, India’s role in CAC48 demonstrates its ability to shape international food safety and quality standards while safeguarding regional interests. By championing standards that are both globally relevant and locally applicable, India reinforces its position as a credible leader in multilateral food governance, enhancing the country’s influence on international trade policy, market access, and agricultural value chains.
– global bihari bureau





