
Researchers Harness Coffee Stains for Ultra-Sensitive Detection
Bengaluru: For many, the sight of a coffee ring left on a table is a familiar, harmless nuisance. Scientists at the city-based Raman Research Institute (RRI), an autonomous institute supported by the Department of Science and Technology, have found a way to turn this everyday phenomenon into a tool for detecting toxic substances in food and water. By studying the so-called “coffee-stain effect,” they have developed a method that could identify dangerous dyes, such as Rhodamine B, at concentrations as low as one part per trillion.
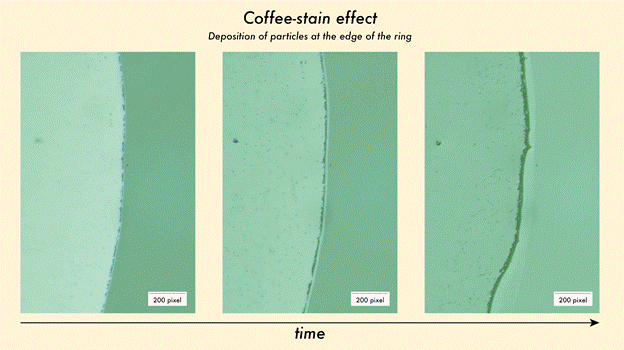
The coffee-stain effect occurs when a droplet of liquid evaporates, pushing suspended particles to its edges and forming a distinctive ring. Researchers exploited this process by depositing water droplets containing gold nanorods—microscopic rods just tens of nanometres in length—on carefully prepared silicon surfaces. As the droplet dried, the nanorods migrated outward, forming rings that vary in density depending on their initial concentration. Denser rings create hundreds of “hot spots” where light is intensified, allowing even minute amounts of Rhodamine B—a toxic dye used in textiles and cosmetics—to produce detectable optical signals.
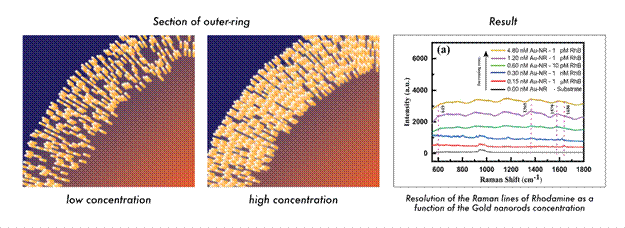
Experiments showed that sparse nanorod rings could detect only relatively high concentrations of the dye, comparable to a drop in a glass of water. Increasing the nanorod concentration enhanced sensitivity dramatically, with the densest rings capable of detecting Rhodamine B down to one part per trillion. In effect, a hundred-fold increase in nanorod density amplified detection sensitivity nearly a million times.
“This method exploits a simple, naturally occurring pattern,” said A. W. Zaibudeen, researcher at RRI. “Rhodamine B is banned in food and cosmetics due to its toxicity, but small quantities can be very difficult to detect. Using this approach, even diluted traces can be identified.” Yatheendran K. M, Engineer B, Soft Condensed Matter, added, “Conventional detection techniques struggle at such low concentrations. By combining the coffee-stain effect with Surface-Enhanced Raman Spectroscopy (SERS), we can achieve far higher sensitivity in a cost-effective manner.”
The practical implications are significant. The technique can be adapted to hand-held Raman spectrometers, making on-site testing in food markets, factories, or water bodies feasible. “The liquid droplet naturally concentrates the nanoparticles along the edges, creating hot spots where even picomolar quantities of toxins can be detected,” said Professor Ranjini Bandyopadhyay, Soft Condensed Matter, RRI. This simple principle transforms a familiar coffee ring into a precise detection landscape.
Beyond Rhodamine B, the team believes the technique could be applied to a wide range of harmful substances, from environmental pollutants to adulterants in food. The approach combines an everyday observation with advanced nanotechnology, offering a practical, inexpensive tool for regulators, laboratories, and small-scale inspectors.
“This research shows that a common, everyday phenomenon—something as ordinary as a coffee ring—can be harnessed to protect public health and the environment,” said Zaibudeen. The team is now exploring ways to refine the method for broader chemical detection, aiming to make it a versatile, accessible technique for monitoring toxins in food and water.
By bridging a familiar kitchen observation with cutting-edge science, the study illustrates how ordinary moments—like watching a coffee drop dry—can be transformed into innovations that safeguard health and the environment, all while remaining remarkably simple and cost-effective.
– global bihari bureau





